Abstract
Introduction
Anticoagulant therapy in individuals with cirrhosis is challenging, as their risk of hemorrhage is significant due to concomitant alterations in primary hemostasis, secondary hemostasis and fibrinolysis. For many years, warfarin and heparin (including low molecular weight heparin) were the only treatment options for patients with thrombosis. Since 2010, novel, direct-acting oral anticoagulants (DOAC) that inhibit either thrombin (dabigatran) or factor Xa (e.g., rivaroxaban, apixaban, edoxaban, and betrixaban) are available. These direct-acting oral anticoagulants do not require laboratory monitoring and have minimal food and drug interactions, which makes them appealing to use for many patients. Unfortunately, there is inadequate data about efficacy and safety of these direct oral anticoagulants in cirrhotic patients as this group was commonly excluded from clinical trials evaluating these medications.
In our study we aim to describe a large single institution experience with use of DOACs in patients with cirrhosis and explore clinical characteristics as potential predictors for bleeding and thrombosis.
Methods:
We conducted a retrospective cohort study of patients with cirrhosis who were seen in our institution between September 1, 2010 and June 30, 2017 and were treated with a DOAC utilizing a database search tool, called the Advanced Cohort Explorer that allows electronic records to be reviewed in a time efficient manner through text search or code search functionality. Only patients with cirrhosis diagnosed by histopathologic evaluation or with clinical presentation consistent with cirrhosis and confirmed by a gastroenterologist or by radiologic imaging (MRI, CT) were included in the study.
Results:
In our study, 106 individuals (male=72) met the inclusion criteria. Ninety three (88%) patients were treated with an anti-Xa inhibitor (rivaroxaban or apixaban), and remaining 13 (12%) with direct thrombin inhibitor (dabigatran). Median age at starting anticoagulant was 66 (range 24-89) years. The most common indication for anticoagulation was atrial fibrillation/flutter (54%), followed by pulmonary embolism/deep vein thrombosis (19%) and splanchnic vein thrombosis (15%). At the time of DOAC initiation, the median Charlson comorbidity index (CCI) was 7 (range 3-15); MELD score was 10 (range 6-24); and platelet count 150,000/ul (range 50,000-432,000). Thirty six (34%) patients had objectively diagnosed varices. Median follow up was 563 days (range: 7-2646).
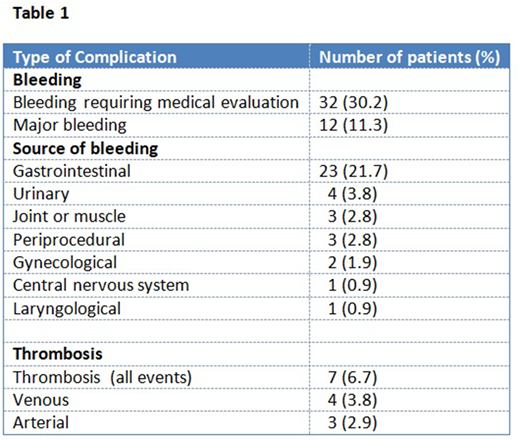
Bleeding requiring medical evaluation occurred in 32 (30.2%) patients. Major bleeding as defined by ISTH was experienced by 12 (11.3%) individuals. The most common source of bleeding was gastrointestinal tract (21.7%); other organs were less commonly affected (table 1). Hemorrhagic complication usually occurred early after starting anticoagulation (median 101 days; range 4-1356), and was the most common reason for discontinuation of anticoagulant treatment (14%), followed by completion of treatment (10%). On Cox proportional hazard modeling, rising BMI (HR: 1.032 per unit increase in BMI; p<0.05) and elevated bilirubin (HR: 1.223 per 1 mg/dL increase) were associated with increased risk of bleeding. Other clinical factors, including sex, type of anticoagulant used, platelet count, PT/INR, albumin, MELD score, varices, portal hypertension, concomitant aspirin use, and Charlson comorbidity index were not associated with bleeding risk.
Thrombotic events while on DOAC affected 7 (6.7%) individuals, which included venous thrombosis in 4 (3.8%) and arterial thrombosis in 3 (2.9%) patients. No clinical factors were associated with increased risk of thrombosis.
Conclusions:
Our study is the largest series of patients with cirrhosis treated with DOACs. Bleeding complications with DOAC use in this population is high. Providers considering starting DOACs in patients with cirrhosis should consider the risk of bleeding in this population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal